|
|
|
A CASE FOR TESTING
Clifford E Carnicom
September 3 2000
A case for the environmental testing of barium and barium
compounds now exists. This case is developed from the following
sequences of events and reasoning:
1. Meteorological study.
2. An anonymous source of information stated to be reliable.
3. Chemistry analysis.
4. pH testing of rainwaters.
5. Physical sample collected in association with aircraft
activity.
6. Testing of chemical hypothesis.
7. Solubility and equilibrium considerations.
8. Environmental testing : water, air, soil.
Each of these topics will now be discussed in greater detail.
1. Meteorological Study:
A reasonable case can be made, based upon meteorological
considerations and observations, that an aerosol particle,
especially of a salt nature, is regularly being introduced into
the atmosphere as a direct result of the unidentified aircraft
operations under consideration. The premise for this case
begins with the meteorological studies of relative humidity at
flight altitude begun in August of 1999 in Santa Fe, NM and
continuing through the middle of the current year.
These studies show the repeated and regular appearance of
cirrus, cirro-stratus and cirro-cumulus cloud deck formations as a
direct result of aircraft operations under conditions of
extremely low relative humidity (avg. 30%). Historic
meteorological observations coupled with reliable sources
demonstrate that such cloud formations are not to be expected,
except under the most unusual of conditions, unless the relative
humidity (with respect to water, per convention and standard
measurement) is greater than 70%. This contradiction is of the
greatest significance, and the rapid, recent and extreme
variation in environmental conditions and activity must be both
explained and accounted for. Observations, on a continuous and
sustained basis since the beginning of 1999, show aircraft as
the source of the materials, having been clearly photographed,
observed, and documented leaving persistent and continuous trails
of an unidentified substance which transforms
itself into the "cloud" formations under the stated conditions
of extreme low relative humidity. The reliable sources referred
to include Vincent Schaefer, inventor of cloud seeding in 1946,
the United States Naval Postgraduate School in Monterey, CA, the
contemporary textbook "Meteorology", by Joseph M. Moran, and a
recent study by both NOAA and NASA. Please refer to the
relative humidity studies elsewhere on this site for further
information on this topic.
In seeking an explanation for this variation, it is helpful to
begin the consideration with the "unusual case" of cloud
formation at relative humidity levels as low as 70%. It is
stated by Schaefer and others that the most likely occurrence of
such cloud formations is best exemplified along the coastline,
where microscopic salt particles, or cloud nuclei, frequently
exist. Such water-seeking nuclei are referred to as
hygroscopic. Therefore, it is observed that the introduction of
hygroscopic nuclei can alter the process of cloud formation to
some degree, although it is seldom to never expected to be
effective under relative humidity levels less than 70%. Most
cloud formation, of any type, is the result of nuclei processes.
Next, it is beneficial to consider the models for cloud
formation, especially cirrus cloud formation, to identify the
most prominent variables that should be considered. Once such
model is presented by Paul J. Demott, at the Department of
Atmospheric Science at Colorado State University. This model
deals specifically with laboratory studies of cirrus cloud
processes. Although any laboratory model is by necessity a
simplification of nature, it remains useful. The primary
variables of the model are temperature, relative humidity, and
aerosol size. Special attention should be given to this last
variable mentioned.
Analysis of this model also results in an important conclusion:
The smaller the size of the nuclei in the atmosphere , the
greater the rate of cirrus cloud formation.
The objective at this stage of the analysis is to identify what
process can be responsible for altering the tenets of
conventional meteorology, and what will provide for repeated cloud
formations under conditions of extremely low relative humidity.
The suggestions given as a result of the above analysis are
twofold: First, it is expected and anticipated that the
material in question delivered from the aircraft is likely of a
salt nature, and second, that it is of an extremely small size.
It is also observed that precipitation seldom accompanies the
cirrus cloud formations that result from the aircraft delivery,
and yet it is a fact that the "clouds" do form. Therefore, the
expectation at this stage is that we are seeking a salt
material, presumed to be extremely small (.e.g., micron, or
sub-micron level quite possible), and that it possesses strong
dessicating, or drying, properties. This latter quality would
explain the apparent contradiction between the frequent
appearance of "clouds" and the associated drought that we find
the country to be currently undergoing. In short, the
introduction of massive amounts of hygroscopic aerosols is
suspected as being one of the major constituents of this program.
2. An anonymous source of information stated to be reliable:
Information has been offered to the public by an anonymous
source in the earlier portion of the year 2000. This source is
simply stated to be reliable to the highest order, and it is
stated that the identity of the source must be protected. This
source states that the material being delivered by aircraft is
composed of barium salts, and that it is being used in
connection with advanced radar studies. No further information
on this aspect of the research is available at this time.
3. Chemistry analysis:
If we postulate that the source of information referenced above
is indeed reliable, it is worthwhile to investigate the
implications of combining the information that has been
presented. It is at least noteworthy to recognize that two
independent sources each make the case of a salt material being
used.
The next stage of this analysis requires an investigation into
barium and barium compounds. I am not a chemist by profession,
but the following information has been acquired:
Barium occurs naturally in two primary forms, barium carbonate
(BaCo3) and barium sulfate (BaSO4). The material is mined from
the earth in these forms. Barium carbonate is commonly known as
witherite, and significant deposits occur in both the United
States and China. There are many other compounds of barium that
can be developed chemically, but this analysis will start with
the simplest case of that which can be mined in abundance and
economically from the earth. Of these two forms of naturally
occurring barium, greater attention has been devoted to barium
carbonate for the following reasons:
1. If barium carbonate is subjected to significant heat, the
combustion process results in the production of barium oxide and
carbon dioxide. It should be mentioned that in all attempts to
determine the actual source of emissions from the aircraft, even
under telephoto conditions, the engines have never been
eliminated from consideration and remain suspect. The fact that
other delivery mechanisms have been observed and recorded does
nothing to interfere with this claim.
2. Barium oxide is a whitish powder.
3. Barium oxide absorbs water, and is used as a dessicant for
that reason.
4. Barium oxide induces respiratory distress, especially
bronchitis.
5. Barium sulfate does not possess these same properties, and is
consequently of less interest at this time.
The first of 5 chemical reactions will therefore be presented.
As I do not make any claim to being a chemist, any errors found
quantitatively or in basic concept to these reactions will be
appreciated.
BaCO3 ->(heat)-> BaO + CO2
The interesting properties of barium oxide (BaO) have been
mentioned. They are especially interesting because they begin
to satisfy the circumstances of meteorological observations and
science, feasible methods of delivery, economics, and formation,
consistent chemical attributes, correlation with observed
patterns of dehydration in the atmosphere, conformal in
appearance, and satisfies at least in part the observed and
reported health affects upon the population.
It is not adequate to stop the investigation at this point. It
is now necessary to devote more attention to the chemistry of
barium oxide, and to learn what is expected if it were released
into or formed within the atmosphere. I offer the following
chemical equations as original work, which will be helpful to
confirm or refute by anyone with further knowledge on this
subject:
Barium oxide combines with water very aggressively. I have the
reaction as:
BaO + 9H2O -> Ba(OH)2 * 8H2O
The resulting compound from this reaction is termed barium
hydrate, or barium hydroxide, octahydate. Barium hydrate exists
as a whitish powder or crystal form.
This reaction explains why barium oxide is used commercially as
a dessicating, or drying agent. It would therefore be expected
to extract the moisture out of the air. If produced at a
sufficiently small size, this reaction goes a long way to
explain the observed alterations in cloud formation under
conditions of extremely low relative humidity. It would also be
consistent with the laboratory model for cirrus cloud formation
mentioned earlier, as well as with the anonymous declaration of
barium salts. Barium oxide is indeed considered to be a salt,
and it possesses a relatively high degree of solubility.
4. pH testing of rainwaters:
If we accept the previous set of events to be from a reasonable
scenario, it is worthwhile to further attempt to validate the
ideas. One such method that can be used to assist in the
process is the pH testing of rainwaters, i.e., the testing for
acidity and alkalinity. This method is suggested because of the
presence of the hydroxides in the reaction above, which
indicates an expected alkalinity that presumably would affect
the rainwaters.
Rainwater samples have been collected on 5 different occasions
in the southern Santa Fe, NM area, and they have been tested for
pH. It should be mentioned that collectable rainwater in the
location mentioned has been an extremely rare event since before
October of 1999 to the present day. Extreme drought is now
characteristic of this location, and the city of Santa Fe itself
is under the next to highest level of water restrictions that
can be imposed under law. As such, collection and ph testing of
rainwater by interested readers is both welcomed and encouraged.
This can be accomplished relatively easily and inexpensively
with pH test kits available at aquarium or pet stores.
The results of this testing are as follows:
June 26 : 6.6
June 27 : 6.6
Aug 17 : 6.2
Aug 18 : 6.3
Aug 19 : 6.6
The average of these tests is 6.46, with a sample standard
deviation of 0.19. The pH scale ranges from 1 to 14, with 1
being extremely acidic and 14 being extremely alkaline.
Distilled water has a pH of 7.0.
The results show that the rainwater samples above are slightly
acidic. These results have caused me some surprise, as my
expectation was that the rainwater should test on the alkaline
side of the scale because of the presence of the hydroxides if
the original hypothesis involving barium carbonate is correct.
At this point, the question was approached in a more open
manner, and the question was rephrased in the following form:
What is the pH of rainwater EXPECTED to be?
The inquiry has resulted in some level of surprise. Two sources
have been located in the research on this question thus far, one
of them being a professor at the University of Hawaii. A
question was posed to the professor in almost exactly the same
form that it arose within my work, and this was:
Why is the rainwater at a low pH, such as 5.5 to 6.5, when the
rivers and lakewaters are showing a pH at or greater than 7.0,
i.e, acidic rainwaters and alkaline groundwaters? The answer
was given that it is actually normal for rainwater to have a pH
of between 5.6 and 5.8. In other words, an acidic quality to
rainwater at this level is expected. This was stated to occur
because of the combination of rainwater with carbon dioxide in
the atmosphere, forming carbonic acid through a perfectly normal
and natural process. Both sources found stated the pH of
rainwater is expected to be at this level, i.e, 5.6-5.8. Acid
rain was stated to be in the class when the pH is less than 5.0.
The conclusion from this investigation, albeit a surprise to
myself, is that rainwater is naturally somewhat acidic.
Considering the results obtained from local rainwater samples
with a pH of 6.5, the new information above now casts a
different and more congruent interpretation. The rainwater
tested locally does show a result which is relatively more
alkaline than the expected values, if the two sources are
presumed to be correct. An explanation for the relatively more
alkaline nature is best explained with the presence of
hydroxides (OH) as supposed in the original hypothesis which led
to the test in the first place.
The results at this stage, therefore, continue to be consistent,
albeit in a surprising manner with respect to pH testing. This
is one reason that it will be helpful for other readers to
investigate the local pH testing of rainwaters across the
country, and to continue to verify the baseline acidic nature
which has been stated by the two sources.
5. Physical sample collected in association with aircraft
activity:
Another stage of testing of the barium carbonate - barium oxide
- barium hydrate hypothesis offered will involve the collection
of physical samples if and when they are available. Reports of
a whitish powder have occurred intermittently throughout the
last two years in association with the aircraft activity, and
have been reported on the message forum. With a single
exception, samples of material of this nature have not been
received by myself.
One sample has been received in August of 2000 which satisfies
the criteria of being a whitish powder. It was collected in
Denver CO on the surface of an automobile after aircraft were
observed emitting continuous trails which subsequently developed
into the common cloud decks. The amount of material collected
was incredibly minute, and exists as a whitish powder or dust.
The amount of material available raised the question as to
whether or not microscopic examination was even possible.
6. Testing of chemical hypothesis:
A microscopic chemical test of the sample referred to above has
been conducted. This test was quite difficult to perform
because of the extremely limited amount of material available,
and the results remain in need of substantiation or refutation.
If indeed there is the unusual presence of a barium compound in
our atmosphere, particularly barium hydrate, it would be
valuable to have a chemical test to help define it's existence.
The following chemical reactions are offered (again, if errors
are found, please notify me):
Ba(OH)2*8H2O + 2 HCL -> BaCl2 + H2(gas) + 9H2O
Ba(OH)2*8H2O + H2SO4 -> BaSO4 + H2(gas) + 9H2O
My research indicates that barium hydrate, if combined with
hydrochloric acid, will form barium chloride, which in turn is
highly soluble in water. Barium hydrate, if combined with
sulfuric acid, will precipitate barium sulfate, a generally
insoluble crystal. These results are expressed with the two
equations above.
Such a test has been conducted with the powdered sample
received. The results would be less ambiguous if more materials
were available for testing, but as it was, the amount available
for each test resided on the sharp end of a needle.
Three trials were performed. Observations in all cases were out
of necessity completed under the microscope due to the extreme
scarcity of the material being analyzed. In each trial, the
whitish powder immediately dissolved in the hydrochloric acid as
hypothesized. In each trial, the whitish powder subjected to
sulfuric acid did result in crystal formations. These crystals
were photographed under the microscope and will be presented on
the web page of this article. The amount of material available
for testing was a critical factor, and the need remains to
continue this testing as the occasion permits. The results of
these tests appear to be consistent with the original hypothesis
that is presented, i.e. barium salts or compounds may now have
an unusual presence in our environment as a result of
aircraft aerosol operations.
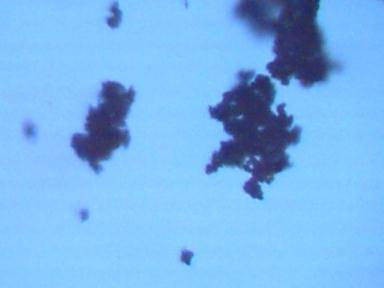
Original White Powder Sample 480x

White powder subjected to sulfuric acid 480x
Crystal formations apparent

White powder subjected to sulfuric acid 480x
Crystal formations apparent
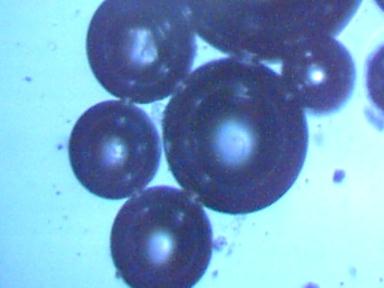
White powder subjected to hydrochloric acid 480x
Dissolves immediately, air bubbles remain.
7. Solubility and equilibrium considerations
There are additional relevant properties of barium compounds,
and the earth alkali elements, of which barium is a member. The
capacity of barium oxide and barium hydroxide to absorb water
appears to be rather striking. Consulting a table of solubility
of salts in water, barium oxide is listed most definitely as a
soluble salt. Furthermore, when ranked with 60 other salt forms
by the solubility constant, barium oxide ranks as number one and
as the most soluble within those listed. The solubility
constant for barium oxide is stated as .0614; this number
outranks the other listings in the table by a factor of hundreds
to thousands to multiples of thousands.
In addition, an intriguing reference has been found that
describes the ability of certain salt forms to absorb water
under varying conditions of relative humidity. Although the
specific case of barium hydrate has not been identified as of
yet, there does appear to be the case of certain salts absorbing
moisture under relative humidity conditions as low as 30%. The
specific case referred to identifies a hydrate form of strontium
chloride at 0deg C. This salt form under these conditions will
absorb moisture under relative humidity conditions of 27%. In
addition, strontium is within the same elemental group as
barium, the earth alkali series. These findings further
substantiate the consideration of barium salts being used in a
dessicating aerosol form, supporting the observations of "cloud"
formation under conditions of extreme low humidity. Attempts
will be made in the future to specifically define the moisture
absorption capacities of barium salt forms with respect to relative
humidity, but the above example demonstrates the feasibility of atmospheric
modifications as have been observed.
[The following information is predictive in nature, and is not
intended for the casual reader. It attempts to predict the equilibrium
constant of the hydrate reaction involved:
If the salt form in question does indeed absorb moisture at
relative humidities of 30% or greater at temperatures of -50deg C.
(flight altitude), then the pressure of the water vapor within the
hydrate form should equal approximately .0143torr. This is based
upon the following:
Pressure of water vapor at -50deg C. is .0477torr (1mb = .750062torr)
Therefore:
P(H2O) / .0477torr = .30
P(H2O) = .0143 torr
P(H20) = 1.882E-5 atmospheres
If the hydrate form is indeed barium hydrate [Ba(OH)2*8H2O]:
Kp (equilibrium constant in atmospheres) = (1.882E-5)^8 = 1.57E-38 atm. at -50deg C.
An important question to now answer is: What is the equilibrium constant, in atmospheres, of the barium hydrate equation that has been hypothesized within this discussion? If reasonable agreement from the actual equilibrium barium hydate chemical reaction with the above calculation is found, then an adequate explanation for the observations recorded has been found. Any assistance from those knowledgeable in the determination of this constant for the reaction specified is appreciated.]
8. Environmental testing : water, soil, air:
A logical case has been developed within this article to
substantiate the need for environmental testing of barium or
barium compounds in our water, air and soil. This case does not
exclude considerations given to additional tests for different
compounds or materials in the future. This case does not
eliminate the need to evaluate other forms of physical material
associated with aircraft operations, such as the sub-micron
fibers or gel samples received and reported. This case does not
exclude the need for further identification of certain
biological components identified within the fibrous materials
mentioned previously.
This case does establish a reasonable requirement and need to
test for barium or barium compounds within our environment based
upon a logical set of events, reasoning, and tests. Barium is
subject to rather stringent environmental restrictions on the
amount permitted in the water supply, e.g.., 2ppm. This case is
dependent upon considerations arising from the science of
meteorology, information sources that are consistent with
observation reports, physics, pH testing and chemistry.
It is recommended that the readership pursue this testing at a
serious and professional level, and that the results be
disclosed to the public at the earliest convenience. Any errors
or revisions in this report will be made as circumstances
require or dictate.
Appreciation is extended to numerous participants on the message
forum that have both initiated and contributed signficantly to this
research topic.
Clifford E Carnicom
Santa Fe NM
Authored at Vallecito Reservoir CO
September 3 2000
Edited September 5 2000